Fast detection of hemorrhagic shock
Biomedical data at the bedside and in the field is abundant, but underutilized. This is particularly true for fast detection of disease onset. In the Auton Lab I investigated the use of bedside monitoring data for fast detection of hemorrhagic shock.
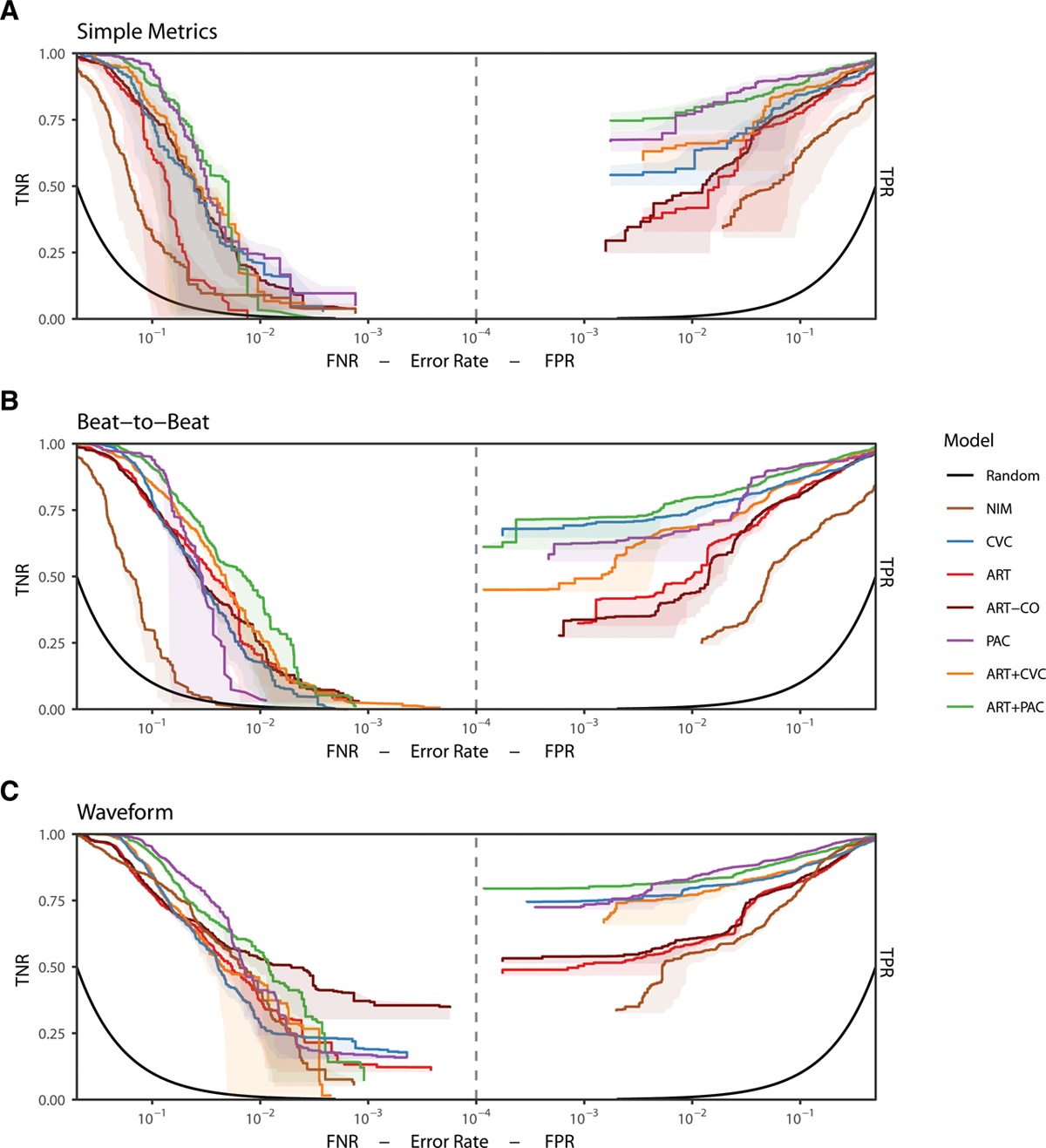
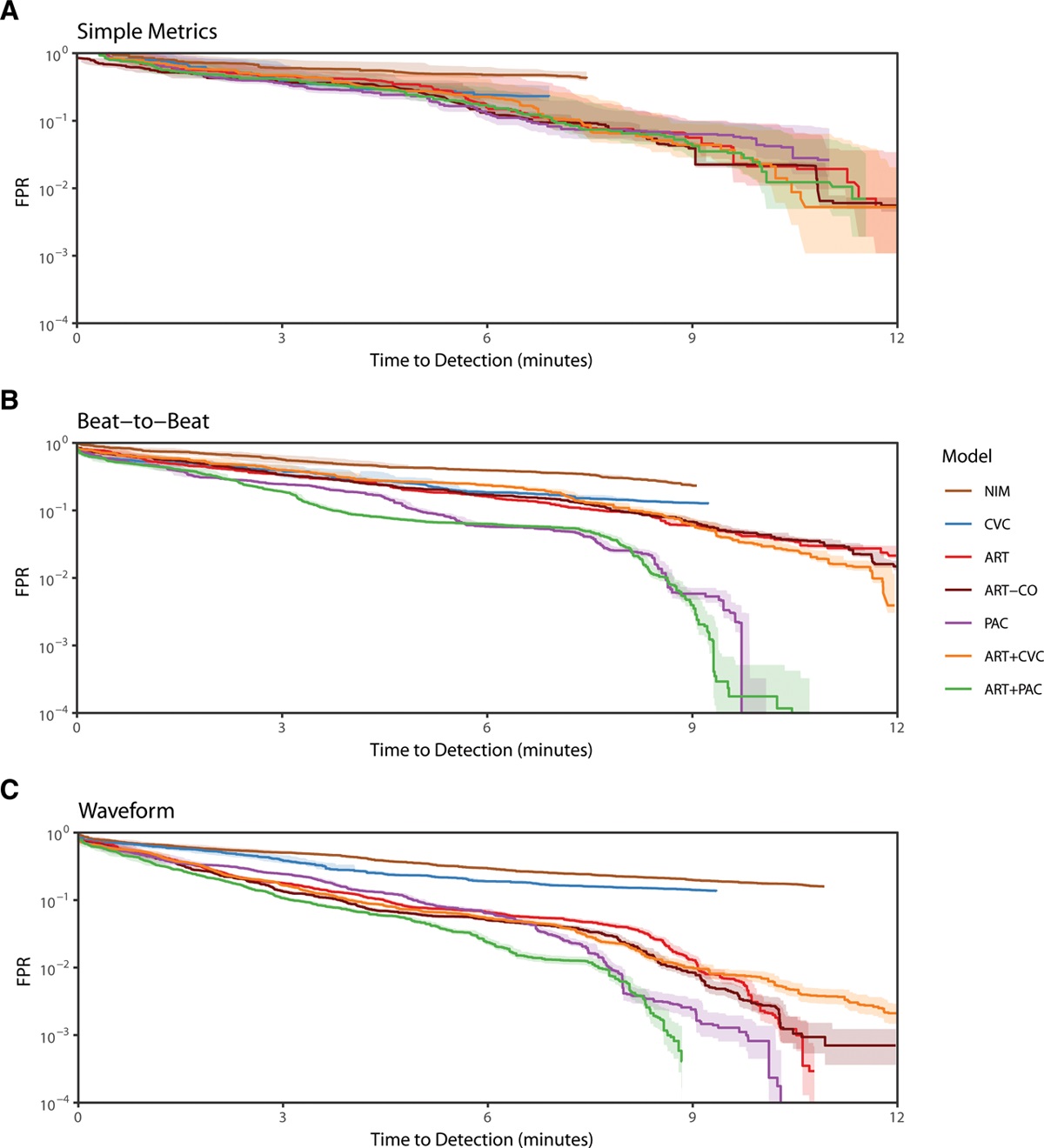
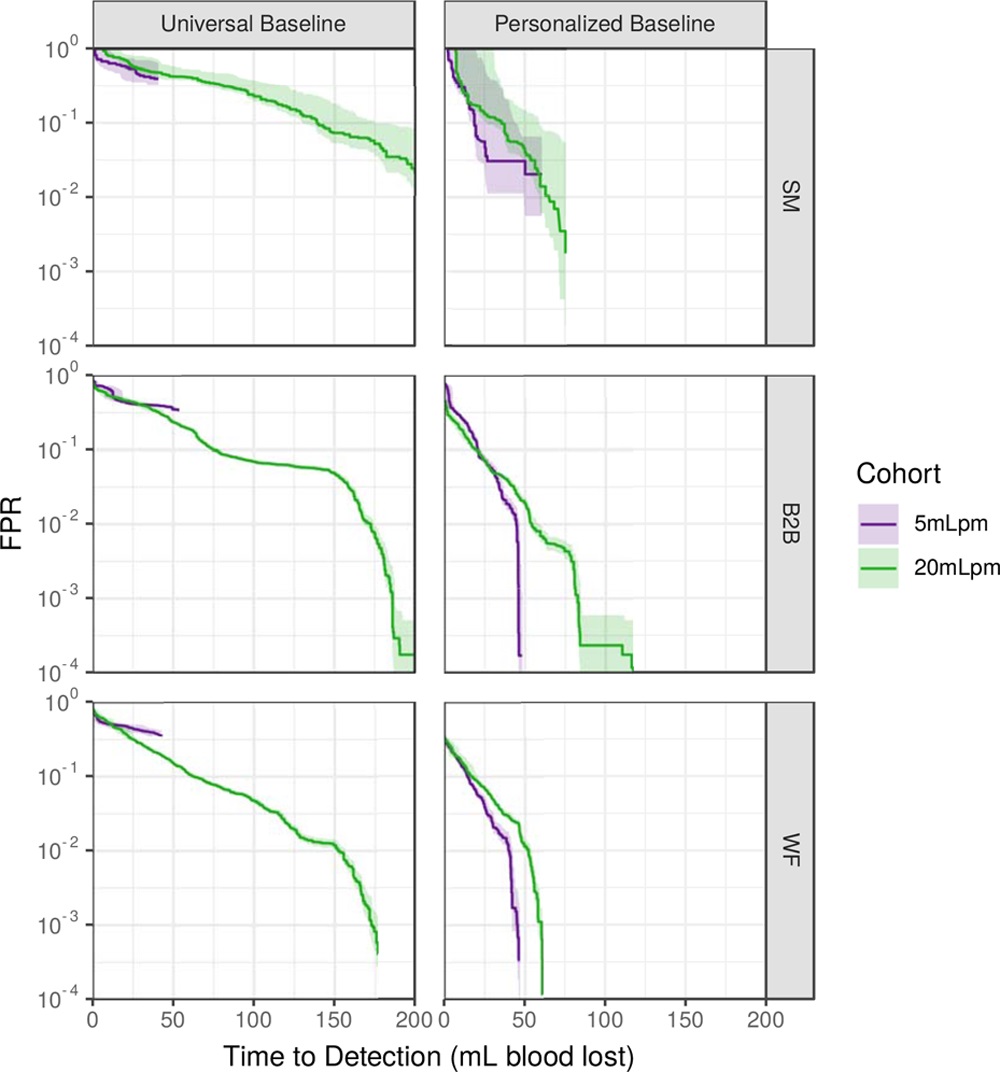
I had the privilege of working closely with many great researchers on this project, both from the Auton Lab and from the University of Pittsburgh Medical Center (UPMC). After taking over the work from Dr. Mathieu Guillame-Bert my main task was train and evaluate machine learning (ML) models of hemorrhagic shock. We used data from prior work (Gómez et al., 2012) collected from animal models of hemorrhage with the objective of quickly detecting the onset of hemorrhage. The main points for investigation were the influence of increasing data density and per-patient personalization (Wertz et al., 2019), along with a look at the sets of more or less invasive monitoring paradigms (Pinsky et al., 2020). Model performance was evaluated based on detection latency using Activity Monitoring Operating Characteristic (AMOC) curves and detection performance using ROCORs, a variant of Receiver Operating Characteristic (ROC) curves. A ROCOR is just two ROC curves glued back-to-back, looking at positive and negative classification performance. If you are not familiar with either of these tools, I encourage you to check out slides 33-54 of a presentation I gave on the topic, which present the two in a step-by-step visual format. That presentation is also helpful for understanding this work in general, as it walks through the results for an audience with no assumed ML background.
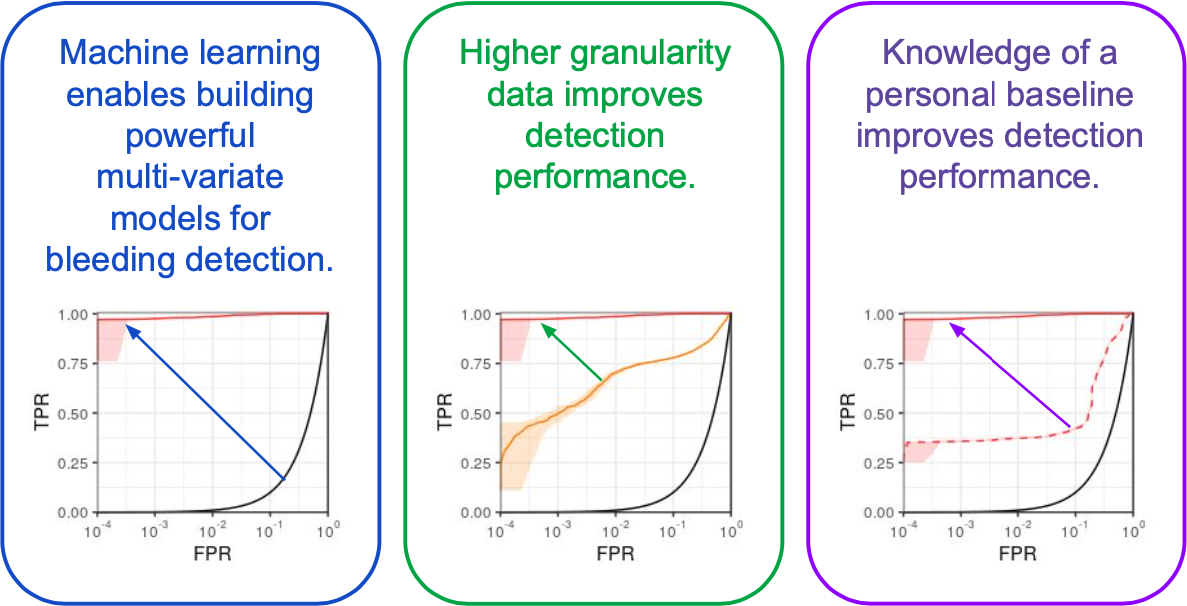
The gory details of the work include all the elements from a standard ML pipeline: raw data wrangling, featurization, model building, and cross-validation. In this way we are able to explore the central questions of the research and provide some guidance on how hemorrhage detection can be accomplished at the bedside, in the field, or even at home. To me, the research really represents an exciting future for medicine where tools built utilize artificial intelligence to give clinical workers better situational awareness and more timely (and useful) alerts. The general findings are summarized as such:
In an extension of this work, I mentored Chufan Gao, a student in CMU’s Robotics Institute Summer Scholars (RISS) program, in approaching the problem from a different, more exploratory direction, trying to understand and cluster the data using deep learning methods instead (Gao et al., 2019).
This work provides a foundation for some of my work on the TRACIR project and spurred some of advancements in data investigation tools, which you can learn more about as well.
- Gómez, H., Mesquida, J., Hermus, L., Polanco, P., Kim, H. K., Zenker, S., Torres, A., Namas, R., Vodovotz, Y., Clermont, G., & others. (2012). Physiologic responses to severe hemorrhagic shock and the genesis of cardiovascular collapse: can irreversibility be anticipated? Journal of Surgical Research, 178(1), 358–369. https://doi.org/10.1016/j.jss.2011.12.015
- Wertz, A., Holder, A. L., Guillame-Bert, M., Clermont, G., Dubrawski, A., & Pinsky, M. R. (2019). Increasing cardiovascular data sampling frequency and referencing it to baseline improve hemorrhage detection. Critical Care Explorations, 1(10), e0058. https://doi.org/10.1097/CCE.0000000000000058
- Pinsky, M. R., Wertz, A., Clermont, G., & Dubrawski, A. (2020). Parsimony of hemodynamic monitoring data sufficient for the detection of hemorrhage. Anesthesia & Analgesia, 130(5), 1176–1187. https://doi.org/10.1213/ANE.0000000000004564
- Gao, C., Falck, F., Goswami, M., Wertz, A., Pinsky, M. R., & Dubrawski, A. (2019). Detecting Patterns of Physiological Response to Hemodynamic Stress via Unsupervised Deep Learning. In arXiv preprint arXiv:1911.05121. https://arxiv.org/abs/1911.05121